Biosimilar Drug Product Development, 1st Edition – FULL INSTANT DOWNLOAD | PDF DOCX EPUB
$84.95 Original price was: $84.95.$32.71Current price is: $32.71.

Name: Biosimilar Drug Product Development
Edition: 1st Edition
ISBN-13: 9780367552497
ISBN-10: 0367552493
Addition ISBN: 9781498718790, 9781315119878, 1498718809, 9781498718806
Author(s): Laszlo Endrenyi; Paul Declerck; SheinChung Chow, Publisher: CRC Press, Copyright: 2017
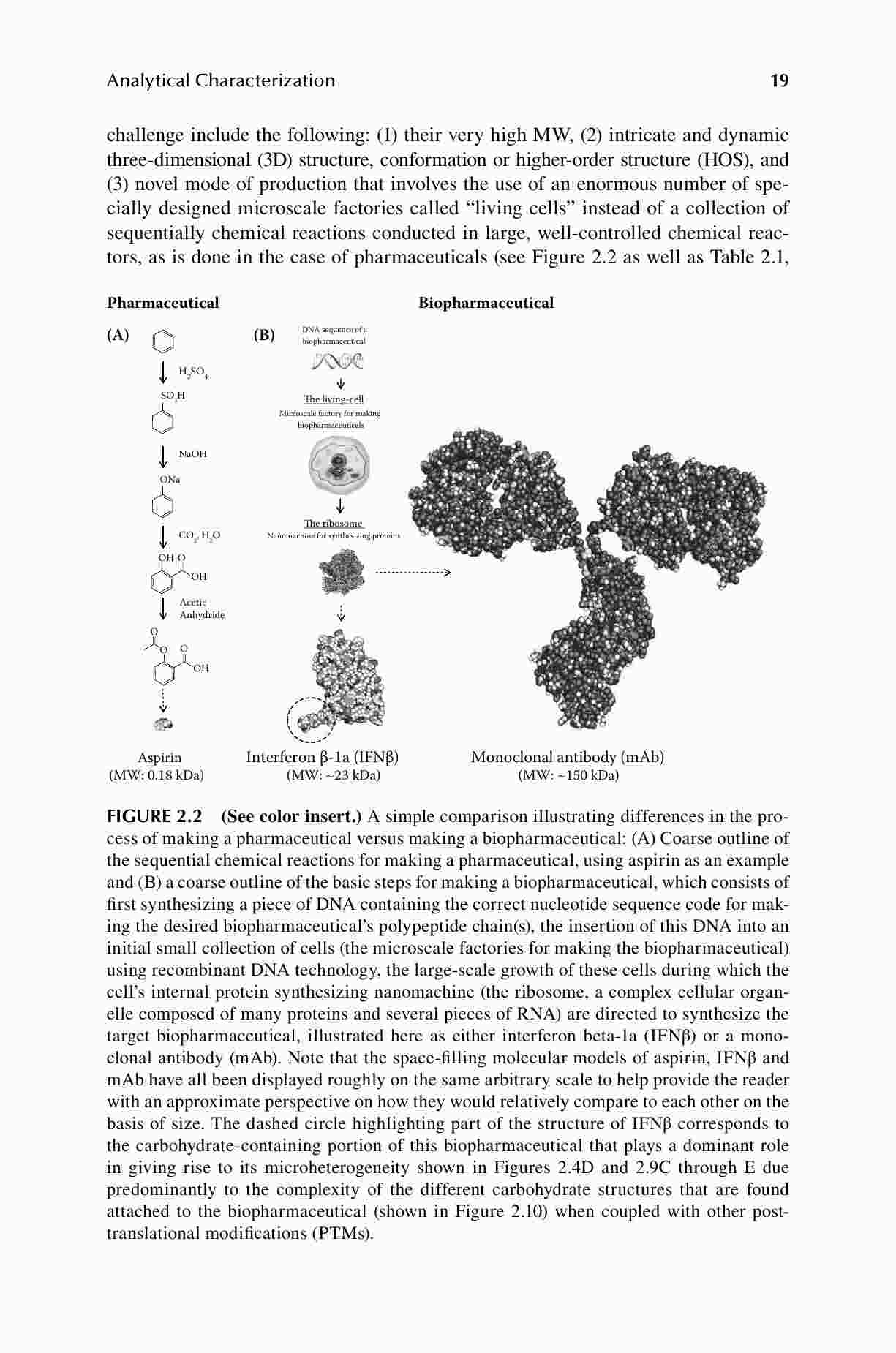
When a biological drug patent expires, alternative biosimilar products are developed. The development of biosimilar products is complicated and involves numerous considerations and steps. The assessment of biosimilarity and interchangeability is also complicated and difficult. Biosimilar Drug Product Development presents current issues for the development of biosimilars and gives detailed reviews of its various stages and contributing factors as well as relevant regulatory pathways and pre- and post-approval issues.
Be the first to review “Biosimilar Drug Product Development, 1st Edition – FULL INSTANT DOWNLOAD | PDF DOCX EPUB” Cancel reply
Related products
Pharmacy
Essentials of Foye’s Principles of Medicinal Chemistry – FULL INSTANT DOWNLOAD | PDF DOCX EPUB
Pharmacy
Mosby’s Drug Reference for Health Professions, 6th Edition – FULL INSTANT DOWNLOAD | PDF DOCX EPUB
Pharmacy
Microbial Biomass Process Technologies and Management – FULL INSTANT DOWNLOAD | PDF DOCX EPUB
Pharmacy
Pharmacotherapy Principles and Practice, 6th Edition – FULL INSTANT DOWNLOAD | PDF DOCX EPUB






















Reviews
There are no reviews yet.